
Next Generation Site-Specific Conjugation and Linker Technologies for ADCs
AJICAP®’s VALUE
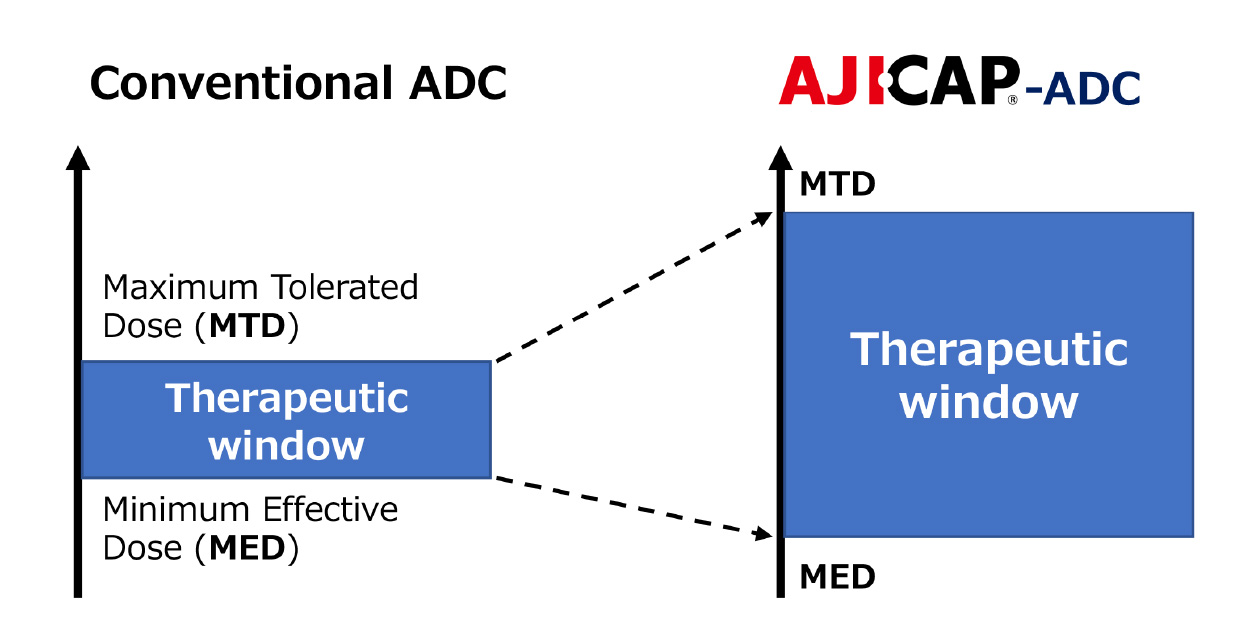
Therapeutic Window Enhancement
- ADCs with higher efficacy and lower toxicity achieved by site-specific conjugation and stable & hydrophilic linker
Straightforward Site-Specific Conjugation Method
- Direct modification on native antibody by chemical method
- No genetic engineering and no enzyme required
- Simple and high yield manufacturing process
- Precisely controlled DAR2 and higher
Applicable for Versatile ADCs
- Site-specific conjugation applicable for any IgG molecule and a variety of payloads
- Stable & hydrophilic linker compatible with various payloads
Index
Technology
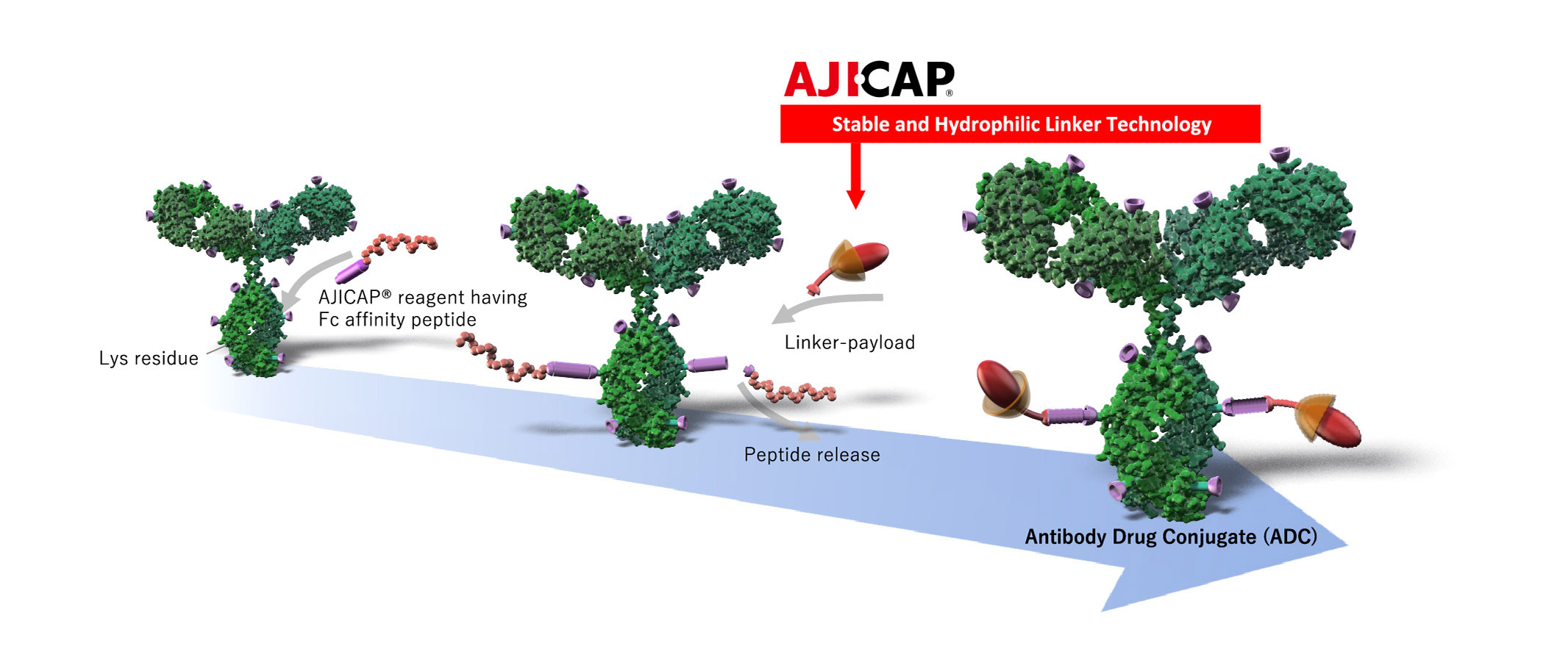
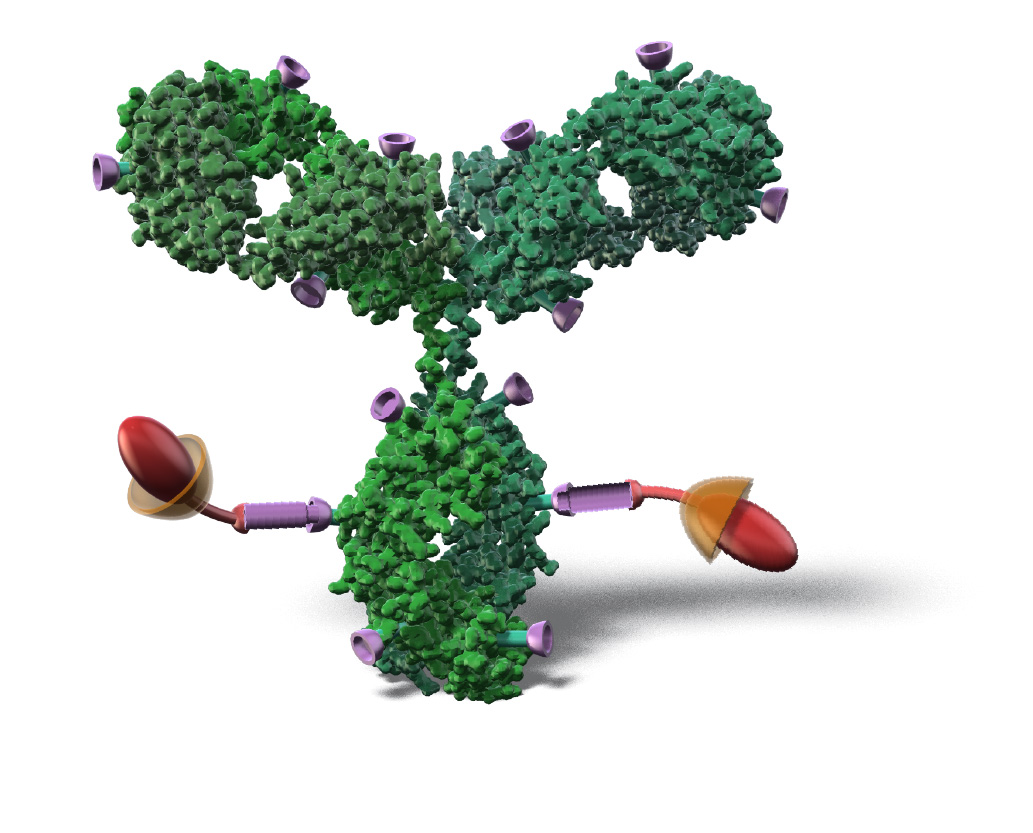
AJICAP® Site-Specific Conjugation
AJICAP® site-specific conjugation is an innovative direct chemical site-specific conjugation method for intact native antibodies, offering significant advantages for next generation ADCs.
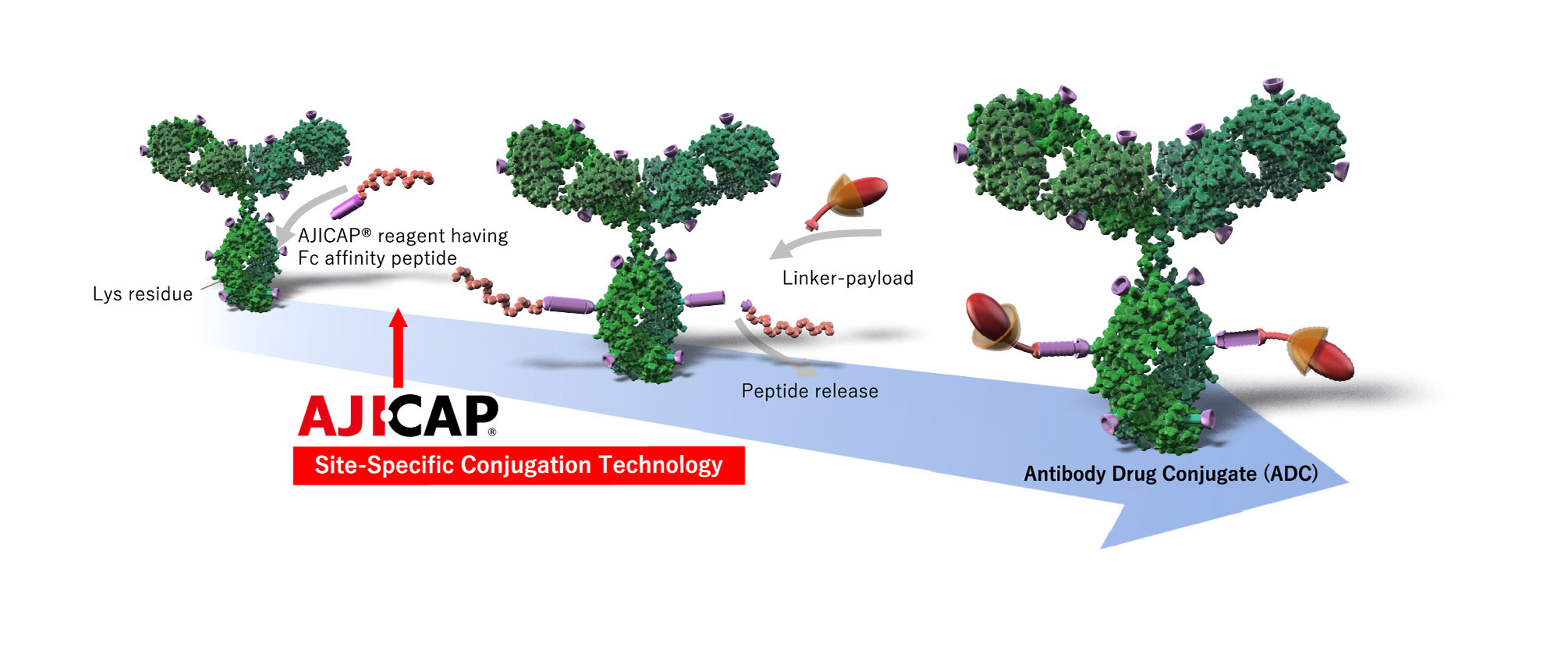
AJICAP® Site-Specific Conjugation consists of:
Introduction of bio-orthogonal functionalities (thiol, azide etc) onto specific Lys residue in Fc region*
Conjugation with
linker-payload
Advantage of AJICAP® Site-Specific Conjugation
Higher efficacy and lower toxicity achieved due to site-specificity
Simple manufacturing and purification process providing CMC advantage
Precisely controlled DAR2 or higher DAR on specific sites
Applicable for a wide variety of antibodies i.e. IgG1, IgG2 and IgG4
Compatible with various payload-linkers i.e. small molecule to large molecule
Application of AJICAP® Site-Specific Conjugation:
ADC platform with various payloads (toxin and others)
In-vivo radio imaging/therapy platform with radioisotopes
Long-acting biopharmaceutical platform for peptides and oligonucleotides
Bispecific platform with proteins

AJICAP® Stable and and Hydrophilic Linker
AJICAP® linker is a novel stable and hydrophilic linker, applicable to various payloads, offering significant advantages for versatile ADCs.
Characteristics of AJICAP® stable and hydrophilic linker
Higher serum stability and controlled release in cancer tissues
Hydrophilic masking of payload
Advantage of AJICAP® Stable and Hydrophilic Linker
Higher efficacy and lower toxicity achieved due to higher serum stability and controlled release in cancer tissues
Dramatically lower aggregation of ADCs by hydrophilic masking of payloads
Applicable for a variety of conjugation technologies including AJICAP® conjugation
Application of AJICAP® Stable and Hydrophilic Linker Technology:
Compatible with a variety of conjugation technologies including:
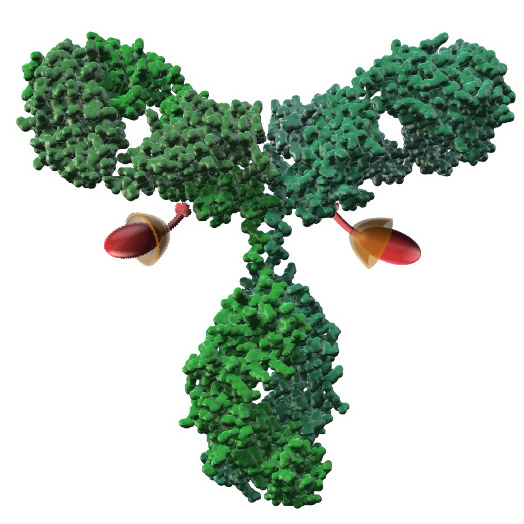
AJICAP® Site-Specific Conjugation Technology
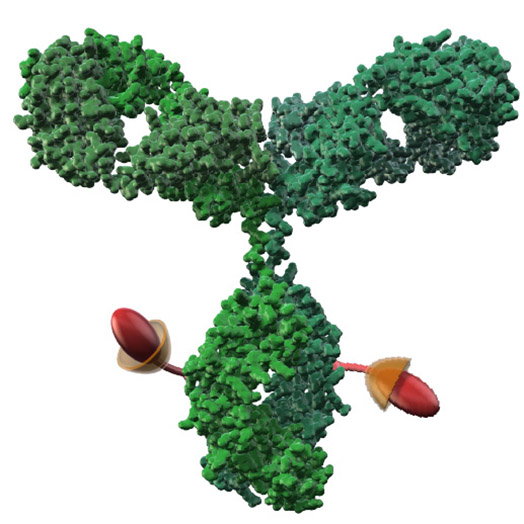
Native Cys conjugation
DAR4-DAR8
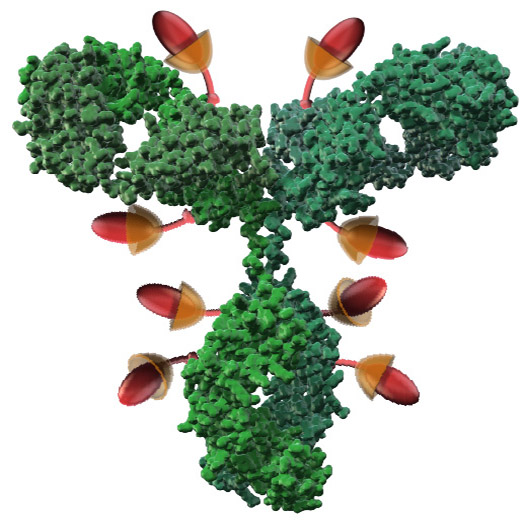
Other genetic engineering and
enzymatic method
Biological Activity of AJICAP®-ADCs
ADCs generated by AJICAP® site-specific conjugation and stable & hydrophilic linker has higher efficacy and lower toxicity.
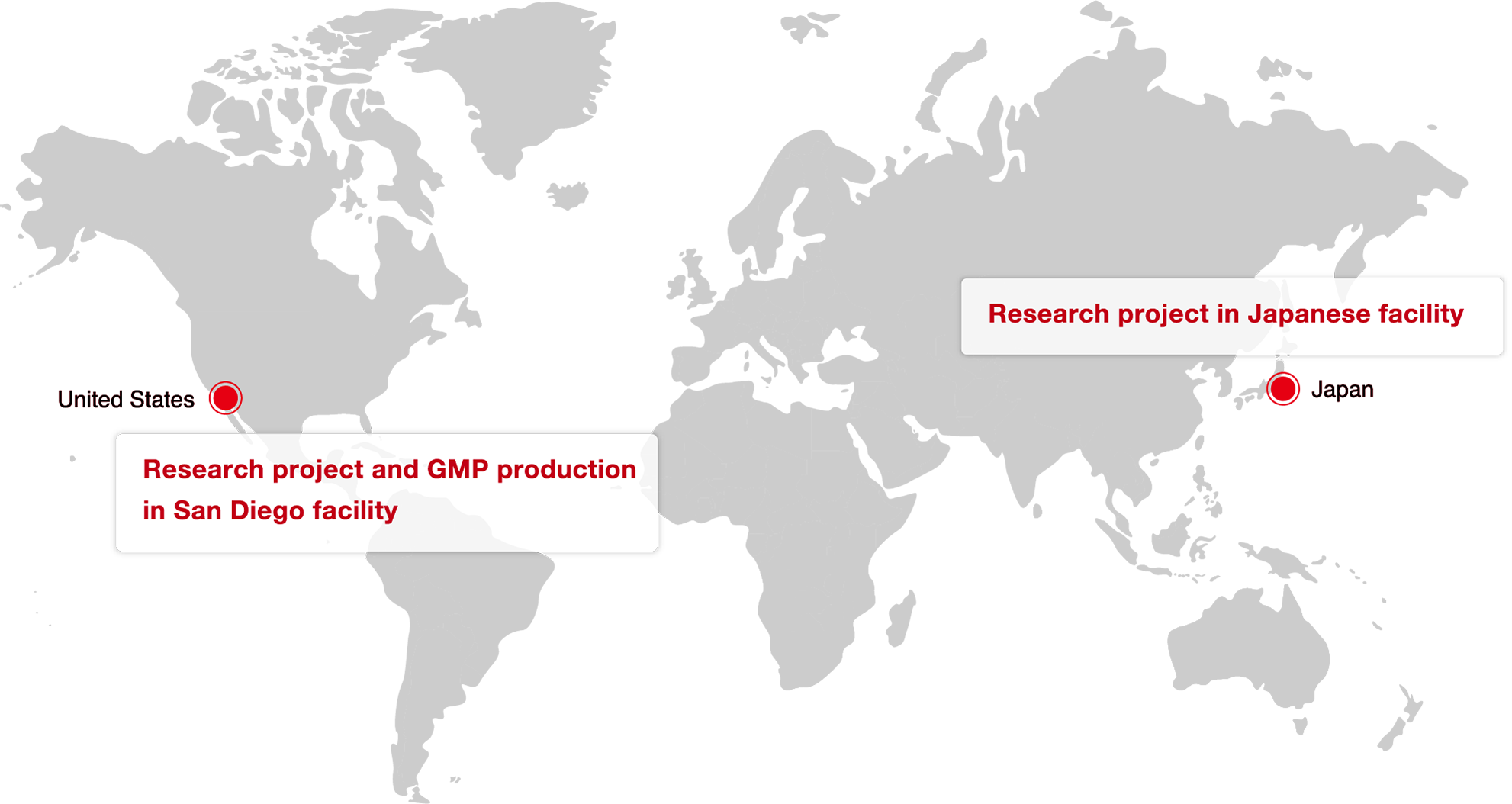
Global Service Offering
Ajinomoto Bio-Pharma Services provides a Global service in Japan and United States.
Service Flow
Service flow of AJICAP® is simple and easy. The following steps outline the process:
Research plan discussion
Client ships antibody (and payload) to Ajinomoto Bio-Pharma Services
Ajinomoto Bio-Pharma Services generates ADCs or other conjugates
Client evaluates ADCs or conjugates in vitro and in vivo
Technology license Go/No Go decision
GxP production discussion
Resources
Resources related to AJICAP® can be found here.
Research Publications
Org. Process Res. Dev. 2023, 27, 6, 1136–1143
The paper described that the AJICAP® second-generation process is a robust and practical approach for the manufacture of ADCs.
Bioconjugate Chemistry, Articles ASAP
The report of AJICAP® second generation, affinity peptide mediated site-specific functionalization that enables the synthesis of next-generation antibody conjugates.
Mol. Pharmaceutics 2021, 18, 4058-4066.
The report of the compatibility and robustness of AJICAP® technology, which enabled the synthesis of a wide variety of ADCs. This is the first report of the therapeutic index estimation of site-specific ADCs produced by utilizing Fc affinity reagent conjugation.
4. AJICAP: Affinity Peptide Mediated Regiodivergent Functionalization of Native Antibodies
Angew. Chem., Int. Ed. 2019, 58, 5592–5597.
The report of AJICAP® first generation, a novel strategy with affinity peptide mediated site-specific functionalization that enables the synthesis of next-generation antibody conjugates.
Anal. Chem. 2019, 91, 12724−12732.
The report of further investigation focusing on comparison of several different analytical methods for drug–antibody ratio (DAR) determination of first-generation AJICAP®-ADC (Angew. Chem., Int. Ed.2019, 58, 5592–5597). The analytical strategy reported here can be applied to the DAR determination of site-specific ADCs.
Org. Process Res. Dev. 2019, 23, 2647−2654.
The report of the first gram-scale ADC synthesis using AJICAP® first generation technology (Angew. Chem., Int. Ed.2019, 58, 5592–5597) employing a scale-down manufacturing approach using tangential flow filtration. The results reported herein for the gram-scale optimization indicate that AJICAP® technology is amenable to relevant manufacturing production scales.
ACS Omega 2019, 4, 20564−20570.
The report of the preparation of site-specific ADCs based on first-generation AJICAP® technology for the utilization in good laboratory practice studies.
Journal of Chromatography B 2020, 1140, 121981.
The report of further investigation focusing on peptide mapping of the AJICAP®-ADC to confirm the exact conjugation position of the first generation AJICAP®-ADC. The analytical strategy described herein demonstrated a robust analytical methodology for revealing the conjugation site of ADCs.
Front. Biosci. (Landmark Ed) 2022, 27, 234.
The report of comparing site-specific maytansinoid-based ADCs synthesized by AJICAP® and T-DM1 in rat safety studies.
J. Am. Soc. Mass. Spectrom. 2020, 31, 1706−1712.
The report of site specific conjugated AJICAP®-ADC Native ion exchange mass spectrometry (SCX-UV-MS) .
Journal of Chromatography B 2021, 1177, 122753.
The report of the chromatographic separation of site-specific ADCs produced by AJICAP® technology using an analytical affinity chromatography HPLC column containing a recombinant FcγIIIa receptor-ligand immobilized on a non-porous polymer resin (NPR).
Contact Us
For further information, please contact us using the link below and an Ajinomoto Bio-Pharma Services representative will contact you.
Story of AJICAP®
For AJICAP® story, please visit.
